Requirements and evolution process of hydrogen fuel cell membranes
Classification:Industrial News
- Author:Luo Xuan
- Release time:Mar-29-2022
【 Summary 】The invention of hydrogen fuel cells can be traced back to 1932, when British engineer Francis first invented hydrogen oxygen fuel cells. And now, with the continuous increase in demand for the enviro
The invention of hydrogen fuel cells can be traced back to 1932, when British engineer Francis first invented hydrogen oxygen fuel cells. With the continuous improvement of environmental and resource demands, hydrogen fuel cell technology has also achieved continuous development. Hydrogen fuel cells have successfully achieved their commercial applications and become the focus of the world's new energy transformation. At present, the components of cation exchange membrane fuel cells (PEMFC) and anion exchange membrane fuel cells (AEMFC) that have received widespread attention usually include bipolar plates, electrodes, catalysts, membranes, and other hardware. Among them, the ion exchange membrane is a very important part, mainly playing a role in selecting permeable ions, separating the anode and cathode, and avoiding battery short circuits.
The ion exchange membrane in hydrogen fuel cells is placed between electrodes containing catalysts, and then sandwiched with two layers of porous gas diffusion layer as the current collector, forming the core component of the fuel cell membrane electrode (MEA). The fuel cell stack is then formed by stacking the membrane electrode and bipolar plate, usually operating at a lower temperature of around 80 ℃. Therefore, the properties and requirements of the ion exchange membrane largely determine the performance of the membrane electrode and fuel cell stack.
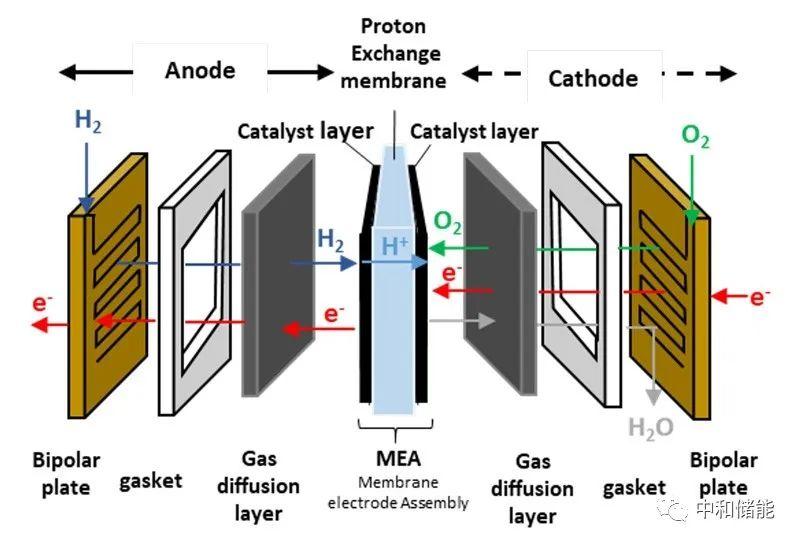
The basic requirements for hydrogen fuel cell membranes include: (1) high proton or anion conductivity to reduce battery impedance and improve battery efficiency; (2) Self insulating to prevent battery short circuit; (3) Good mechanical properties can continuously block the reaction gas; (4) No defects, low hydrogen and oxygen transmittance; (5) High chemical stability, able to maintain good properties during service life under redox, acidic or alkaline conditions. (6) In addition, to achieve large-scale and widespread promotion and application, its processing and production costs should also be as low as possible. At present, proton exchange membranes are mainly used in fuel cells, while anion exchange membranes are still in the initial and research stages. However, the use of non precious metal catalysts accompanying them is of great significance for the development of fuel cells.
For proton exchange membranes in fuel cells, their development can be traced back to the early 1960s. The earliest proton exchange membrane fuel cells invented by GE used phenolic resin sulfonic acid membranes or polystyrene sulfonic acid membranes. Polystyrene sulfonic acid membranes were obtained by sulfonating styrene monomers immersed in a polyvinylidene fluoride matrix and their cross-linked monomers. This type of membrane did not go far due to serious aging problems. In 1966, DuPont invented the Nafion membrane, also known as perfluorosulfonic acid membrane (PFSA). This membrane is based on the copolymerization reaction between tetrafluoroethylene and perfluoroethylene ether monomers containing sulfonyl fluorine in the side chain, followed by hydrolysis transformation to obtain - SO3H, which is then produced. Major international fuel cell proton membrane manufacturers such as DuPont in the United States, Xunitro in Japan, and Xuhua Chengdu produce perfluorosulfonic acid proton exchange membranes. And according to DuPont's product information table, the proton conductivity of its Nafion proton exchange membrane in liquid water at 25 ° C has exceeded 0.083 S/cm, indicating excellent conductivity compared to similar products.
The thickness of the membrane used in fuel cells is usually less than 50 microns, which makes the membrane have high conductivity and good ion transport performance. The smaller the thickness, the more difficult it is to produce. In 1969, Gore Company first discovered expanded polytetrafluoroethylene (ePTFE), a material that produces an ideal microporous structure during expansion, giving it excellent properties such as high strength to weight ratio, high mechanical properties, high heat and corrosion resistance, and began to be used in its product production. In 1995, Gore Company compounded perfluorosulfonic acid resin with expanded polytetrafluoroethylene polymer through impregnation drying on the basis of DuPont Nafion membrane production,
Production of enhanced Gore Select perfluorosulfonic acid composite film reduces thickness, significantly reduces the amount of perfluorosulfonic acid resin used, and achieves significant cost reduction. The Gore Select composite enhanced proton membrane has more outstanding conductivity, chemical stability, and mechanical strength, and its thickness can reach up to 5 μ m. Currently, it has achieved mass production with a thickness of 8 μ m. It is very suitable for proton exchange membranes in automotive fuel cells and is also the most advanced enhanced membrane today. The implementation of this ultra-thin proton exchange membrane can effectively reduce ion conduction resistance, reduce Ohmic polarization, enhance water vapor conductivity, increase proton conductivity, and enhance the power density of the fuel cell stack.
Other companies have also begun to try producing composite reinforcement films to improve their performance in fuel cells. Japanese Asahi seeds used 2.7 wt% PTFE around 2000
The original fiber has developed a reinforcement film with a thickness of 50 μ m, and DSM Solutech uses non fluorinated ultra-high molecular weight polyethylene as a porous matrix reinforcement material to produce a thickness of 25
The μ m enhanced film has shown good performance. In China, Dongyue Future Hydrogen Energy and Kerun New Materials have achieved independent production of proton exchange membranes, with products as thin as 15 μ m and 12 μ m. Guodian Hydrogen Energy has reduced the thickness of proton membranes to 8 μ m. Although there is still a certain gap with foreign first-class advanced technologies, it is rapidly developing. According to relevant reports, the first phase of Dongyue Future Hydrogen Energy's proton exchange membrane production line with an annual output of 1.5 million square meters has been officially put into operation. The Kerun 1 million square meter proton exchange membrane project has also started construction, and the Wuhan Green Dynamic Hydrogen Energy's proton exchange membrane production line with an annual output of 300000 square meters under State Grid Hydrogen Energy Corporation has also been completed and put into operation. We can believe that with the continuous promotion of the fuel cell industry, the localization of proton exchange membranes is expected in the future.
Related articles:
Development scale and future prediction of fuel cell and membrane markets
Hydrogen storage technology supports the development of hydrogen energy storage in the field of "new energy generation+fuel cell power generation"
Technology and Market Overview of Ion Exchange Membranes in Liquid Flow Cells and Fuel Cells

