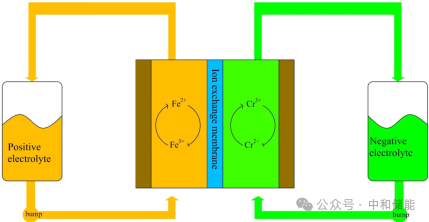
In recent years, the iron chromium flow energy storage battery system represented by "Ronghe No.1" has received widespread market attention due to its lower electrolyte cost compared to all vanadium flow. This article elaborates on the research and improvement directions of iron chromium (electrolyte, electrode, separator, and battery structure) for reference by readers.Iron chromium batteries use Fe2+/Fe3+pairs and Cr2+/Cr3+pairs as positive and negative active substances respectively, usually with hydrochloric acid as the supporting electrolyte [1], as shown in the schematic diagram. During the charging and discharging process, the electrolyte enters the two half cells through a circulating pump. The Fe2+/Fe3+and Cr2+/Cr3+pairs undergo oxidation-reduction reactions on the electrode surface, respectively. The electrons released from the positive electrode are transferred to the negative electrode through an external circuit. Inside the battery, ions move in the solution and exchange protons with the ion exchange membrane, forming a complete circuit, thereby achieving the mutual conversion of chemical energy and electrical energy.
铁铬电池示意图
Iron chromium battery is the earliest liquid flow battery technology that emerged. It was included in NASA's research program as early as 1974 and received support from the US Department of Energy. In 1978, iron chromium batteries were successfully developed with Fe2+/Fe3+and Cr2+/Cr3+pairs as positive and negative active materials, respectively. A 1kW/13kWh iron chromium battery stack was developed and applied to photovoltaic energy storage systems. Subsequently, with the support of the "Moonlight Project" in Japan, the Japanese company successfully developed 10kW and 60kW iron chromium battery stacks through technological breakthroughs in key materials such as batteries. Later, Enervault Company in the United States built a 250kW/1MWh iron chromium battery energy storage power station in 2014. At present, State Grid Corporation of China has also built a 250kW/1.5MWh iron chromium flow battery energy storage demonstration power station, which will further promote the application and promotion of flow batteries, especially iron chromium battery technology. In recent years, domestic and foreign researchers have also conducted extensive basic research on iron chromium battery technology, such as electrode optimization and design, electrolyte system optimization, catalyst screening, battery structure design and optimization, further developing iron chromium flow batteries.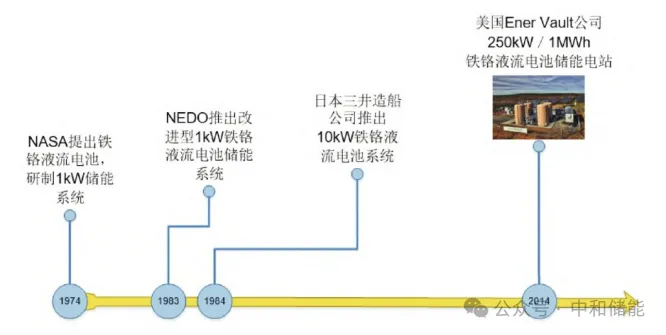
Research and application history of ferrochrome flow batteries abroad[8]
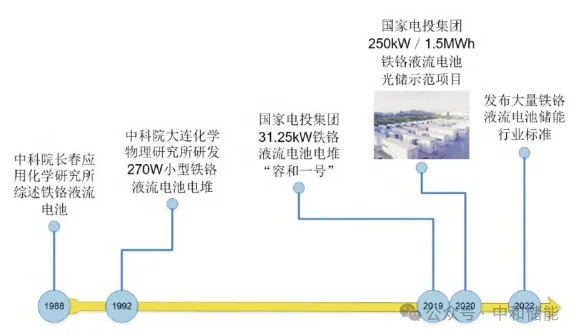
Research and application history of domestic ferrochrome flow batteries[8]
For electrode design, carbon materials have also become the most widely used electrode materials for flow batteries. NASA was the first to study the electrochemical behavior of Cr2+/Cr3+pairs and hydrogen evolution reactions on metal and carbon electrodes (such as carbon paper, carbon cloth, carbon felt, etc.), and proposed loading Au/Pb on the surface of carbon felt to promote the redox reaction of Cr2+/Cr3+pairs [2]. However, after increasing the temperature, Au can cause serious hydrogen evolution reactions. Therefore, Bi was introduced as a catalyst to improve the performance of iron chromium batteries [3]. The Japanese Electrical Engineering Laboratory screened various carbon materials and found that the thermal decomposition polyacrylonitrile based carbon fiber cloth had a good effect. The material was used as an electrode to prepare a 1kW battery stack. Subsequently, carbon materials were used as the matrix for activation treatment, and chemically modified polyacrylonitrile based carbon fiber felt was used as the electrode to develop a 10kW iron chromium battery stack, reducing the introduction of precious metal catalysts [4]. Chen N et al. obtained a surface loaded SiO2 graphite felt electrode by heat treatment of graphite felt soaked in silica acid, effectively increasing the electrode specific surface area and active sites, promoting the reaction activity of chromium ions. The energy efficiency of the battery can reach about 80% at a current density of 120mA cm-2, which is 8.2% higher than that of the untreated electrode[5]。
For the separator material, the most suitable separator material for iron chromium batteries currently is the Nafion series perfluorosulfonic acid proton exchange membrane produced by DuPont. Sun CY et al. further investigated the physical and chemical properties of Nafion series separators (212, 115, and 117) in the electrolyte of iron chromium batteries, as well as the energy efficiency, capacity decay rate, and electrolyte utilization rate of assembled batteries. Through comprehensive evaluation of the three separators, it was found that Nafion 212 separator is currently the most suitable separator for iron chromium battery applications [6]. Although Nafion 212 perfluorosulfonic acid proton exchange membrane has the best performance, its high material cost leads to high battery costs, which to some extent limits its development. To reduce battery costs, Sun CY et al. prepared low-cost polyether ether ketone membranes with different sulfonation degrees and compared their performance with Nafion 115 membranes treated with hydrogen peroxide and sulfuric acid. After testing, batteries prepared with SPEEK separators have lower self discharge rate and capacity decay rate, and relatively higher Coulombic efficiency. According to the calculation of a 1MW/8MWh iron chromium battery energy storage system, the proportion of diaphragm cost can be reduced from 39% to 5%, significantly reducing the system cost[7]。 Compared to other liquid flow battery systems, the electrolyte is the core point of iron chromium batteries, which directly determines their energy storage cost. At present, the poor electrochemical activity, easy aging, hydrogen evolution reaction, fast capacity decay, and low energy efficiency of Cr3+ions in the electrolyte of iron chromium batteries still limit their commercial development. There are many studies aimed at improving the electrochemical activity of Cr3+and solving aging problems. In the hydrochloric acid aqueous solution of CrCl3, there are three complex ions: Cr (H2O) 63+, Cr (H2O) 5Cl2+, and Cr (H2O) 4Cl2+. However, Cr (H2O) 63+does not have chemical activity, and the other two ions will undergo transformation and aging. Research has found that increasing the temperature of the electrolyte to 65 ℃ can promote the conversion of inactive Cr (H2O) 63+to active Cr (H2O) 5Cl2+, thereby solving the aging problem of the electrolyte. Moreover, the increase in temperature promotes the electrochemical reaction rate to a certain extent, but the increase in temperature also reduces the selectivity of the separator to a certain extent, causing cross contamination of the electrolyte [3]. Cheng DS et al. proposed using N-alkylamines as an additive in chromium chloride solution to alleviate the aging problem of the electrolyte to a certain extent. Zhang Lu et al. found that certain organic amines (such as ethylenediamine or 1,4-butylamine hydrochloride) or ammonium chloride were used as additives in the CrCl3-HCl electrolyte system, which can effectively improve the storage performance of Cr2+/Cr3+pairs and make them have good electrochemical reaction activity, thereby solving the aging problem of the electrolyte in iron chromium batteries. They also proposed using ammonium chloride instead of hydrochloric acid medium in the iron chromium battery system as a supporting electrolyte. The experimental results show that Cr3+ions can form stable chloroammonium coordination compounds in the CrCl3-NH4Cl electrolyte system, preventing the electrolyte from aging. Moreover, adding 0.1M hydrochloric acid as an additive in this system can further improve the redox reversibility of the electrolyte and does not cause significant hydrogen evolution reactions.(四)Battery structure and system The development of iron chromium flow batteries has also gone through decades of progress, and battery efficiency continues to improve. A significant development in battery structure is the improvement of flow field channels. The further development of flow field channels greatly promotes the efficiency of iron chromium and other liquid flow batteries. Zeng et al. proposed a snake shaped flow field and a cross shaped flow field, which change the flow of electrolyte by carving channels on bipolar plates. This can effectively shorten the flow distance of electrolyte in porous electrodes, reduce the flow resistance of electrolyte in porous electrodes, and make the electrolyte more evenly distributed throughout the electrode area. Compared with traditional structures, it greatly reduces impedance and improves efficiency. For the battery system, as early as 1992 in China, a research conducted by Dalian Institute of Chemical Physics, Chinese Academy of Sciences, used polyacrylonitrile carbon felt as electrode, electrodeposited bismuth as negative electrode electrocatalyst and hydrogen evolution inhibitor before the operation of the battery, polystyrene sulfonic acid type cation exchange membrane as diaphragm, and wax impregnated graphite plate as bipolar plate to assemble a 270W iron chromium flow battery system. Tests have shown that the battery system performs stably over several charging and discharging cycles, with a current efficiency of 93%, a voltage efficiency of 78%, and an energy efficiency of 72% [10]. At the end of 2020, State Grid Corporation of China successfully trial produced "Ronghe No.1" ®” The high-capacity battery stack was successfully applied in the Zhangjiakou Zhanshigou 250kW/1.5MWh demonstration project in Hebei Province, which was also the world's largest iron chromium flow battery system at that time.In summary, iron chromium flow batteries are mainly affected by unfavorable factors such as poor electrochemical activity of Cr3+ions, easy aging, hydrogen evolution reaction, fast capacity decay, and low energy efficiency. Currently, domestic iron chromium batteries have already had photovoltaic storage demonstration projects in 2020, and research on electrodes, electrolytes, separators, and high-power batteries will still be the focus of this direction. It is believed that there will be further development in practice and research in the next decade.
Reference:
[1]Su, Yang & Chen, Na & Ren, Hai-lin & Guo, Li-li & Li, Zhen & Wang, Xiao-min. (2022). Preparation and Properties of Indium Ion Modified Graphite Felt Composite Electrode. Frontiers in Chemistry. 10. 10.3389/fchem.2022.899287.
[2]Swette, L, & Jalan, V. Development of electrodes for the NASA iron/chromium redox system and factors affecting their performance. United States.[3]Gahn, R F, Hagedorn, N H, & Ling, J S. Single-cell-performance studies on the Fe/Cr redox energy-storage system using mixed-reactant solutions at elevated temperature. United States.[4]林兆勤,江志韫.日本铁铬氧化还原液流电池的研究进展 电池材料和结构及子系统的研宄概况[J].电源技术,1991,6:3240.[5]Alotto, Piergiorgio & Guarnieri, Massimo & Moro, Federico, 2014."Redox flow batteries for the storage of renewable energy: A review," Renewable and Sustainable Energy Reviews, Elsevier, vol. 29(C), pages 325-335.[6]Sun, Chuanyu & Huan, Zhang. (2019). Investigation of Nafion series membranes on the performance of iron‐chromium redox flow battery. International Journal of Energy Research. 43. 10.1002/er.4875.[7]Sun, Chuanyu & Huan, Zhang & Luo, Xu-Dong & Chen, Na. (2019). A comparative study of Nafion and sulfonated poly(ether ether ketone) membrane performance for iron-chromium redox flow battery. Ionics. 25. 10.1007/s11581-019-02971-0.[8]朱昊天,谢小银,白恩瑞等. 铬铁氧化还原液流电池与其电极改性的研究 [J]. 电源技术, 2023, 47 (11): 1394-1398.
[9] LIU Q H,GRIM G M,PAPANDREW A B,et al.High performance vanadium redox flow batteries with optimized electrode configuration and membrane selection[J].Journal of the Electrochemical Society,2012,159(8):A1246-A1252.[10]衣宝廉,梁炳春,张恩浚等.铁铬氧化还原液流电池系统[J].化工学报,1992,(03):330-336.