Latest Progress Unveiled: Iron-Sulfur Battery Achieves Breakthrough in Ultra-High Current Density of 120 mA/cm², Reaching Economic Advantage Crossover Point
Classification:Industrial News
- Author:ZH Energy
- Release time:Dec-01-2025
【 Summary 】The iron-sulfur flow battery achieves an energy efficiency of 67.9% at a current density of 120 mA/cm² and exhibits excellent stability over 1000 cycles.

Recently, ZH Energy, in collaboration with the team from Huaneng Clean Energy Research Institute and Central South University, has made a significant breakthrough in the field of anode catalytic materials for iron-sulfur flow batteries. They loaded iron phthalocyanine (FePc) onto graphite felt and used it as a high-efficiency electrocatalyst for the S₄²⁻/S₂²⁻ redox reaction. The iron-sulfur flow battery catalyzed by this FePc achieves an energy efficiency of 67.9% at a current density of 120 mA/cm² and exhibits excellent stability over 1000 cycles. Recently, the relevant results were published in the Journal of Power Sources under the title “Boosting the electrochemical properties of polysulfide by iron(II) phthalocyanine and its mechanism”.
Research Background
With the growing global energy demand and increasingly severe environmental issues, developing new clean energy sources and supporting energy storage technologies has become a top priority. Iron-sulfur (S/Fe) flow batteries have shown broad prospects in large-scale energy storage due to their advantages of high safety, environmental friendliness, and low cost. However, the slow reduction kinetics of polysulfides during charging severely limits the energy efficiency and power density of the battery. Aiming at this key issue, this study developed a high-efficiency electrocatalyst based on iron phthalocyanine (FePc), which significantly improves the overall performance of iron-sulfur flow batteries.
Material Characterization and Performance Testing
Through XRD, infrared spectroscopy, and Raman spectroscopy, it was confirmed that FePc was successfully synthesized by the reaction of phthalonitrile and anhydrous FeCl₃. SEM images show that FePc is uniformly distributed on the surface of carbon fibers as fine particles, forming an effective catalytic interface.
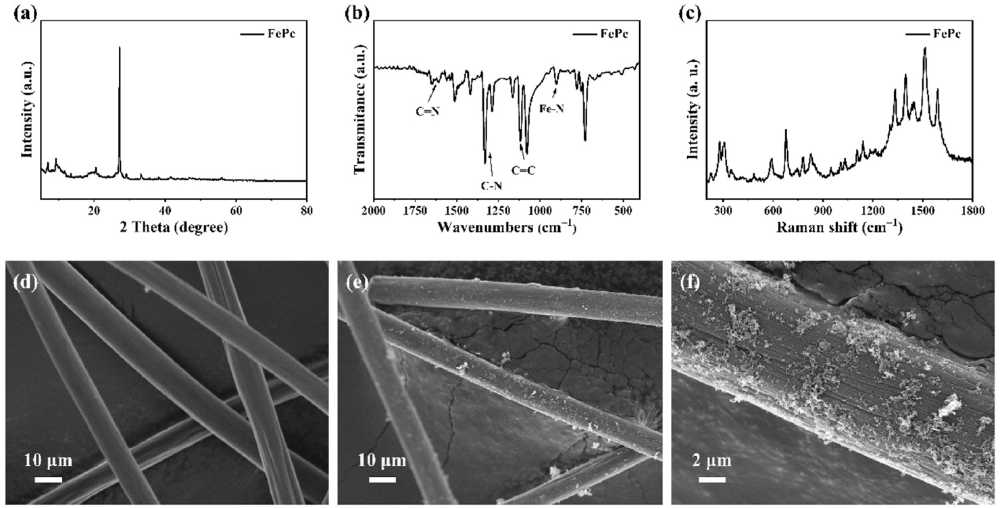
Figure 1. (a) X-ray diffraction (XRD) pattern of FePc, (b) Fourier-transform infrared (FTIR) spectrum of FePc, (c) Raman spectrum of the synthesized FePc, (d) Scanning electron microscopy (SEM) image of carbon fibers in the blank electrode, (e), (f) SEM images of FePc-loaded carbon fibers at different magnification levels.
Cyclic voltammetry (CV) tests show that the redox peak potential difference of the FePc-modified electrode decreases from 0.64 V to 0.23 V, and the peak current increases significantly.Through electrochemical double-layer capacitance (EDLC) tests, the double-layer capacitance (Cdl) value of the FePc electrode is 4.40 mF/cm², which is higher than 3.75 mF/cm² of the blank electrode, indicating an enlarged effective reaction area. The charge transfer resistance of the FePc electrode is 6.55 Ω/cm², much lower than 34.8 Ω/cm² of the blank electrode. The Tafel slope decreases from 388.6 mV/dec to 76.5 mV/dec, and the exchange current density increases from 0.004 mA/cm² to 0.163 mA/cm², significantly improving the polysulfide ion reaction kinetics.
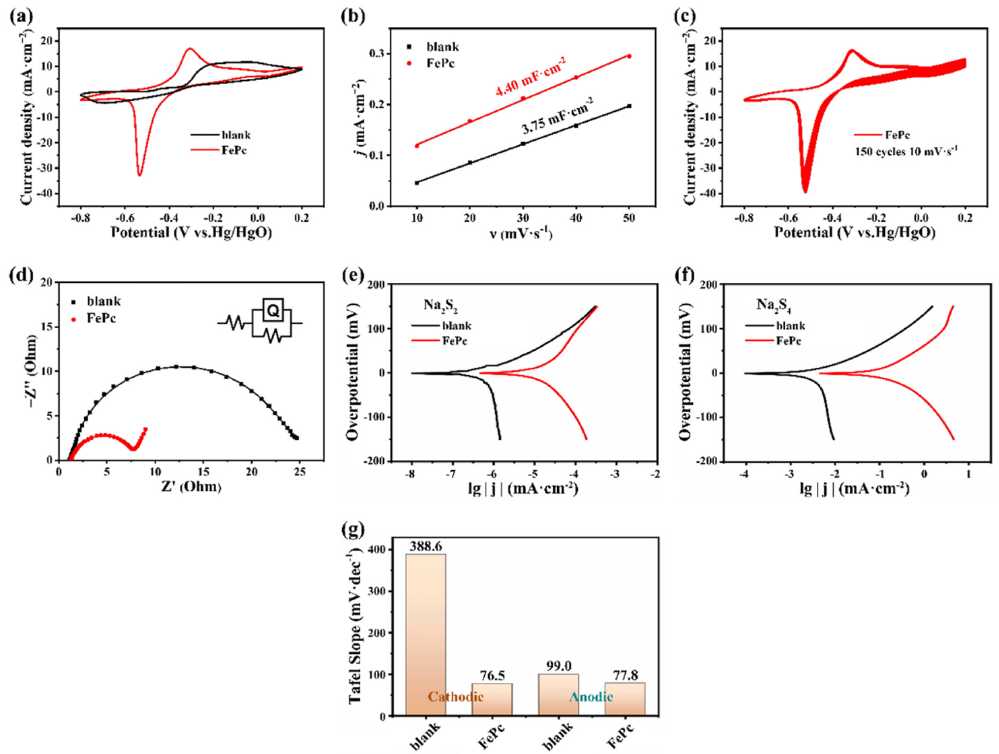
Figure 2. (a) Cyclic voltammetry (CV) curve of FePc (scan rate: 10 mV/s), (b) Double-layer capacitance (Cdl) of FePc, (c) CV curve of FePc after 150 cycles, (d) Nyquist plots of the blank electrode and FePc-modified electrode, (e) Tafel curves of the blank and FePc-loaded electrodes in Na₂S₄ solution, (f) Comparison of Tafel curves between the blank and FePc-loaded electrodes, (g) Comparison of Tafel slope values between the blank and FePc-loaded electrodes in polysulfide solution.
Galvanostatic intermittent titration technique (GITT) tests show that the internal resistance voltage drop of the FePc-based battery is only 0.053 V, much lower than 0.337 V of the blank battery. In the current density range of 20–120 mA/cm², the FePc-based battery exhibits higher energy efficiency across the board: it exceeds 75% at 80 mA/cm² and remains at 67.9% even at 120 mA/cm². The peak power density of the FePc-based battery reaches 206.4 mW/cm², significantly superior to 147.9 mW/cm² of the blank battery. After 1000 cycles, the capacity retention rate reaches 96.8%, and the capacity fading rate is only 0.00214% per cycle.
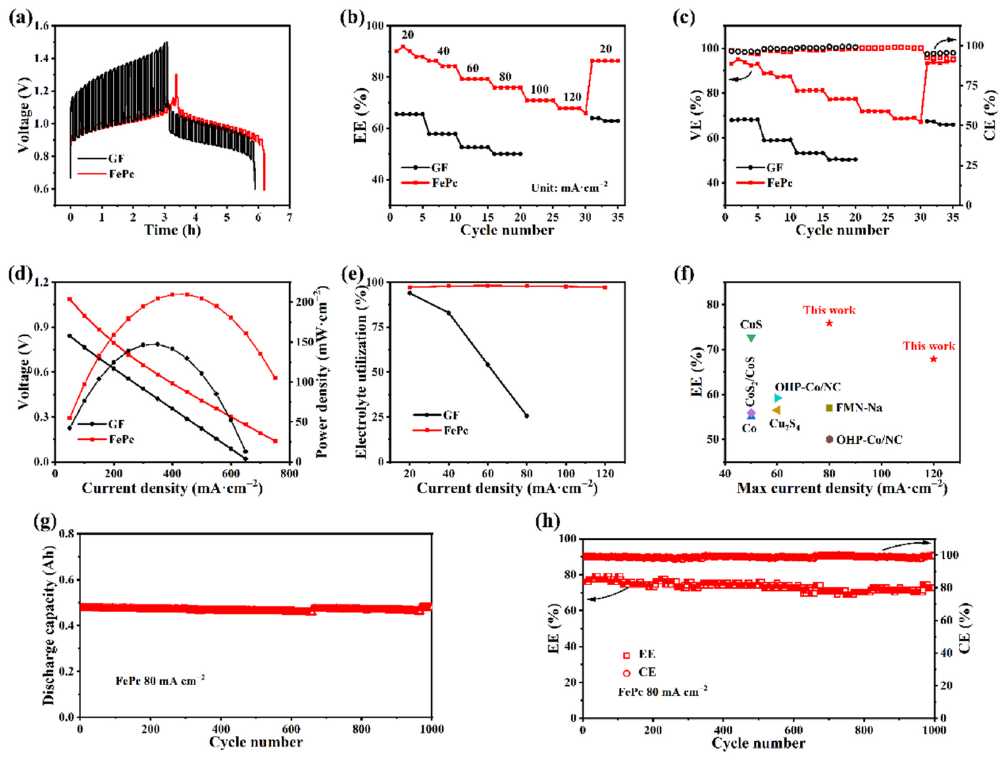
Figure 3. Iron-sulfur flow batteries using C and FePc as the anode. (a) Galvanostatic intermittent titration; (b) Rate capability of the battery at different current densities; (c) Voltage efficiency and coulombic efficiency of the battery; (d) Discharge voltage and power density at different current densities; (e) Electrolyte utilization rate; (f) Performance comparison between this study and other literature-reported levels; (g) Long-cycle discharge capacity plot of the battery; (h) Long-cycle energy efficiency plot of the battery.
DFT calculations reveal that polysulfides preferentially adsorb onto the central iron atom of FePc: the intermediate sulfur species are more stable, sulfur bonds tend to elongate, the free energy barrier is reduced, and the rate-determining step of the reaction shifts from “electrochemical reaction” to “electrochemical desorption”, thus achieving high-efficiency catalysis.
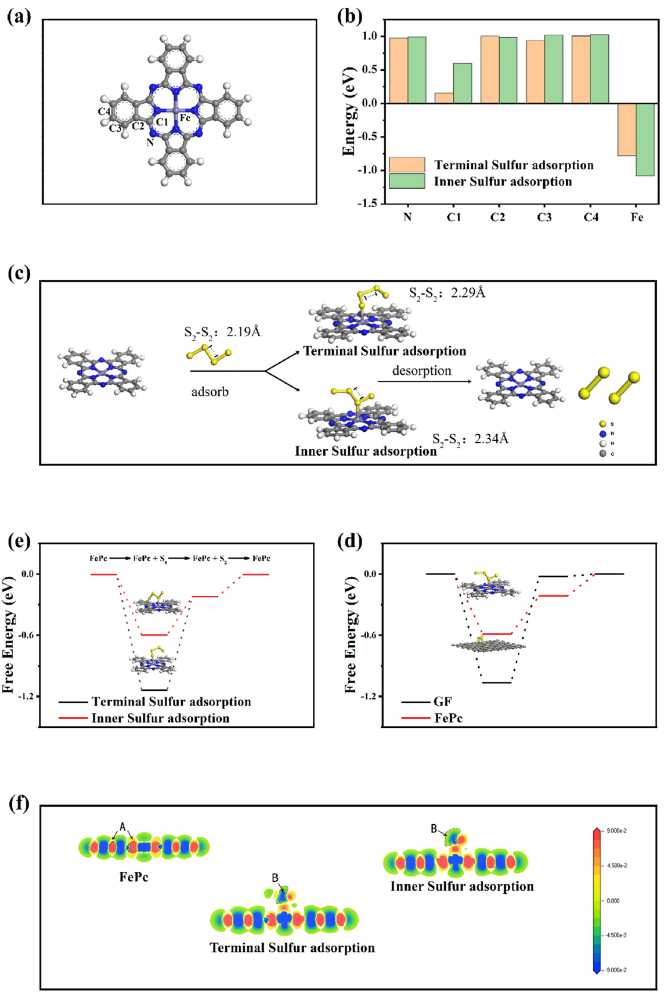
Figure 4. (a) Potential adsorption sites on FePc. (b) Adsorption energies of two adsorption types at different sites; (c) Changes in Gibbs free energy during the two adsorption processes; (d) Schematic diagram of the two adsorption modes and bond length variations; (e) Free energy changes during the adsorption of polysulfides on FePc and graphite felt (GF); (f) Charge difference density of different adsorption modes.
Summary and Outlook
This study confirms that iron phthalocyanine (FePc) is a high-efficiency catalyst with both high activity and high stability in iron-sulfur (S/Fe) flow batteries. It can significantly mitigate the kinetic bottleneck of polysulfide redox reactions, and maintain excellent energy efficiency and long-term cycle life even under high-rate operating conditions.In addition, this study clarifies the catalytic mechanism of Fe-N₄ active sites in the S/Fe system at the molecular level for the first time. It reveals that FePc promotes polysulfide conversion through a threefold mechanism: stabilizing intermediates, reducing energy barriers, and accelerating electron transfer — providing an important theoretical basis for designing next-generation low-cost, high-efficiency flow battery catalytic systems.At the application level, as an easily accessible and low-cost organometallic molecule, FePc not only enhances the operational stability and cycle durability of the battery, but its performance has also reached the international advanced level. It offers a reliable technical path and solution for the practical engineering application of long-duration energy storage technology.
Previously, the iron-sulfur flow battery system jointly developed by ZH Energy, Central South University, and Huaneng Clean Energy Research Institute has successfully passed on-site acceptance. The first iron-sulfur flow battery system for the power generation side is scheduled to be deployed at Huaneng Clean Energy Research Institute. With the advantages of high safety and low cost, it meets the needs of new energy integration, has gained authoritative recognition from the power generation sector, and contributes to the construction of the new-type power system.

To obtain the full paper, please contact us!
Further Reading:
A New Era! First Iron-Sulfur System for Power Generation Side to Be Deployed at Huaneng Clean Energy Research Institute
Iron-Sulfur Flow Battery with Enhanced Energy Density via Prussian Blue Solid
Copper Sulfide Electrocatalyst via Successive Ionic Layer Adsorption and Reaction (SILAR) for Iron-Sulfur Batteries
High-Performance Membrane and Electrode Materials for Sulfide-Ferricyanide Flow Batteries

